The octet rule is described as a simple rule of thumb that states that atoms with 8 electrons in their outer shell are stable. Select one or more.
Sulphur has 6 valance electrons and 6 fluorine atoms also donate 6 electrons.

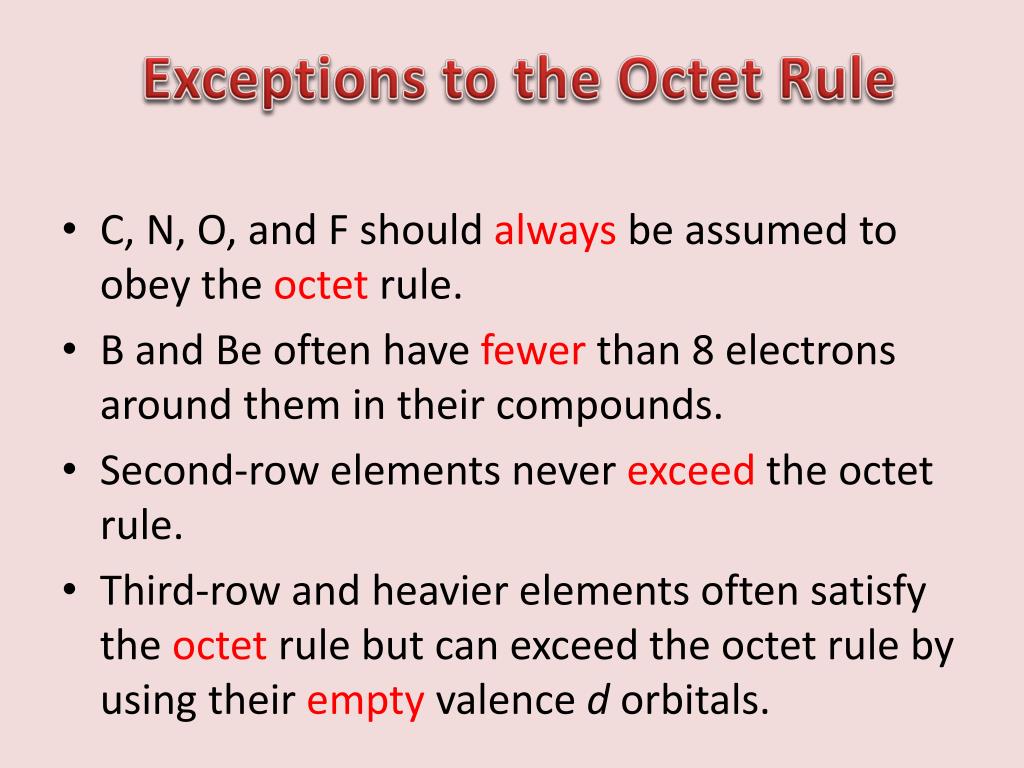
. The two elements that most commonly fail to complete an octet are boron and aluminium. In general the elements that obey this rule include the s-block elements and the p-block elements except hydrogen helium and. Additionally which follows octet rule.
Not yet answered I Points possible. What elements follow octet. I know I can eliminate E because the total.
A NF3 b CF4 c SF4 d PH3 e HCl I know I can eliminate A and B because nitrogen and carbon follow the octet rule. Chlorine and sulfur will not strictly follow the octet rule. As the octet rule requires eight electrons around each atom a molecule with an odd number of electrons must disobey the octet rule.
100 In the Water of Hydration lab what is the. However many atoms below atomic number 20 often form compounds that do not follow the octet rule. Not yet answered 1 Points possible.
Since 1p subshells do not exist some elements find stability in 1s 2 configurations. The two elements that most commonly fail to complete an octet are boron and aluminum. Nitrogen dioxide is the chemical compound with the formula NO 2.
How does oxygen form an. Elements that do not follow the octet rule. Draw the Lewis structures for the following molecules or ions for which the octet rule is not obeyed.
Chlorine and sulfur will not strictly follow the octet rule. Some elements that disobey the octet rule include. This is the best answer based on feedback and ratings.
The molecules of the halogens oxygen nitrogen and carbon are known to obey the octet rule. Elements like hydrogen lithium helium do. Hydrogen has only one valence electron and only one place to form a bond with another atom.
They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule. The octet rule is a rule in chemistry where elements want to form bonds to attain 8 electrons in their valence shell. The atoms of these elements can sometimes expand their octet by utilizing the d-orbitals found in the third principal energy level and beyond.
The octet rule states that atoms below atomic number 20 tend to combine so that they. The compound actually exists but it is highly reactive that is to say unstable. Save for oxygen nitrogen carbon and fluorine most other elements dont follow.
Boron has three valence electrons. There is persistent radical character on nitrogen because it has an unpaired electron. Which one of the following compounds does not follow the octet rule.
Does no2 follow octet rule. Elements that do not follow the octet rule. Calculating the formal charge on each.
The only possibility for boron is to bond to three hydrogen atoms in which case it forms a compound borane BH3 that does not fulfill the octet rule. Compounds following the octet rule must have 8 electrons in the valence shell of their central atom. The octet rule states that atoms below atomic number 20 tend to combine so that they.
100 Which elements do not strictly follow the octet rule when they appear in the Lewis structure of a molecule. The central atom is in bold. Molecules with unpaired electrons are termed free radicals.
The octet rule states that for atoms to be stable they must have eight electrons on their outermost shells. Elements like hydrogen lithium helium do not obey the octet rule. Hydrogen Fluorine Sulfur Carbon Chlorine Oxygen Question 32 Status.
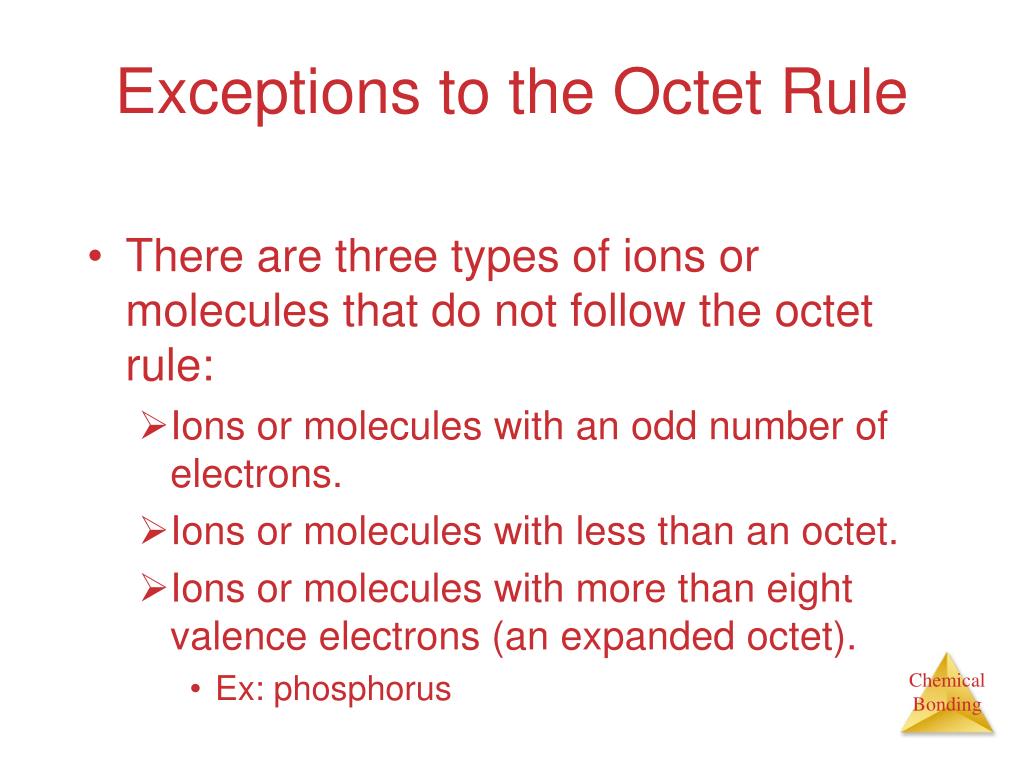
The total number of valence electrons is 52617. The octet rule is a chemical rule of thumb that reflects the observation that elements tend to bond in such a. Key Takeaways The Octet Rule and Its Exceptions.
The Lewis structure of a molecule has a high probability of violating the octet rule if 1. Which of these do not obey the octet rule. What element does not follow the octet rule.
Another exception of the octet rule is transition elements. They can only lose or gain one electron to become stable due to which they follow the octet rule. The octet rule is only applicable to the main group elements.
On the other hand some elements exhibit hypervalency and have the ability to form hypervalent molecules. In general the elements that obey this rule include the s-block elements and the p-block elements except hydrogen helium and lithium. Explaination Expanded octet can be seen in atoms that have vacant orbital this is an essential requireme View the full answer.
This rule is not strictly followed by some elements such as sulphur and chlorine. The molecules of the halogens oxygen nitrogen and carbon are known to obey the octet rule. Chlorine and sulfur will not strictly follow the octet rule.
Beryllium has only two valence atoms and can form only electron pair bonds in two locations. List Four Elements that do not Obey the Octet Rule. See full answer below.
Elements like hydrogen lithium helium do not obey the octet rule. Hydrogen Fluorine Sulfur Carbon Chlorine Oxygen Question 32 Status. There are also a.
Select one or more. Hydrogen beryllium and boron have too few electrons to form an octet. Does BF3 have a complete octet.
The octet rule is only applicable to the main group elements. Hydrogen beryllium and boron have too few electrons to form an octet. Molecules through an odd number of electrons are fairly rare in the s and also p blocks but rather common.
General exception to the octet preeminence include molecule that have an odd number of electrons and molecules in i m sorry one or an ext atoms possess much more or fewer 보다 eight electrons. The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons and thus the electron configuration of a noble gas. Free Radicals Some elements most notably nitrogen can form compounds that do not obey the octet rule.
Another exception of the octet rule is transition elements. What is octet rule and give two examples which do not follow this rule. Again nitrogen dioxide does not follow the octet rule for one of its atoms namely nitrogen.
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 5318726

Which Of The Following Compounds Does Not Follow The Octet Rule Youtube
Ppt Exceptions To The Octet Rule Powerpoint Presentation Free Download Id 3528141
0 Comments